| Test Capacity |
Real time PCR |
| Detection Channel |
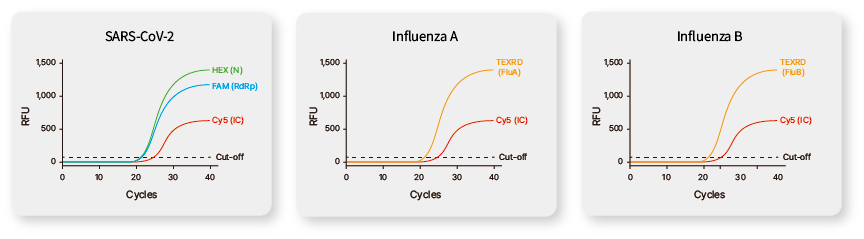
FAM for SARS-CoV-2 RdRp gene , HEX for SARS-CoV-2 RdRp gene , Texas Red for Influenza A & B, Cy5 for Internal Control |
| Heating / Cooling rate |
96 tests/kit |
| Operation System |
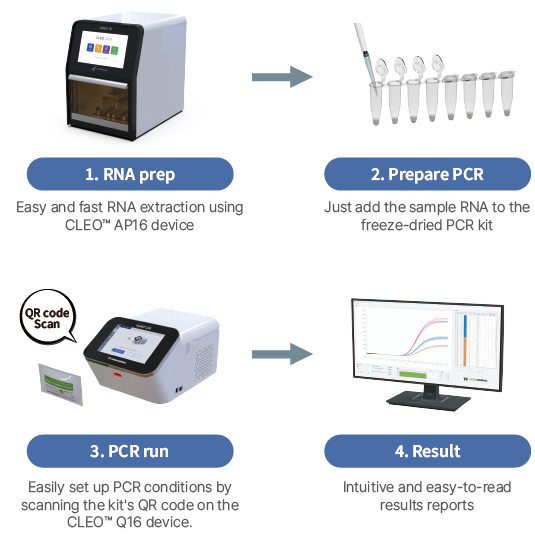
Lyophilized (Freeze-dried) in PCR tube |
| Detection |
100 copies/rxn |
| Temperature Accuracy |
Respectively detect the reference specimen of high and low concentrations in different time ranges-10 times, and the precision values of intra-assay and inter-assay Ct values were all <1.0%. |
| Test ID and Sample ID Input |
For analytical specificity, cross-reactivity was evaluated by testing related pathogens and microorganisms that are likely to be present in the cervical, vaginal, and genital. The test was repeated three times and showed no cross-reactivity. |
| Display |
Oropharyngeal, nasopharyngeal swabs and sputum specimens |
| Input power/Max power |
Shipping and Storage at room temperature for 12 months |
| Dimensions |
CLEO Q16 (Wizbiosolutions), CFX96 (Bio-Rad), QuantStudio™ 5 (ThermoFisher) |