| Principle of Procedure |
Real-time PCR |
| Target |
IS6110 and mbp64 gene of Mycobacterium tuberculosis (MTB) |
| Tests for Package |
100 Tests/kit |
| Reagent Type |
Premix type |
| Limit of Detection (LoD) |
2 copies of MTB genome |
| Precision |
Respectively detect the reference specimen of high and low concentrations in
different time ranges-10 times, and the precision values of intra-assay and
inter-assay Ct values were all <5%. |
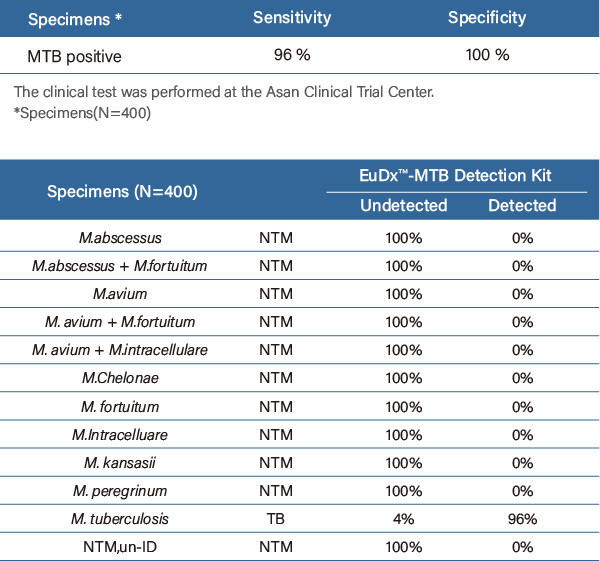
| Specificity |
Sensitivity: 100% (20/20)
Specificity: 100% (30/30) |
| Sample Types |
Human sputum, urine, tissue, blood, and cultured samples |
| Shipping/Storage |
Storage at -20 ºC for 18 months |
| Compatible Instruments |
CLEO™ Q16 (Wizbiosolutions), CFX96(Bio-Rad), ABI7500 |