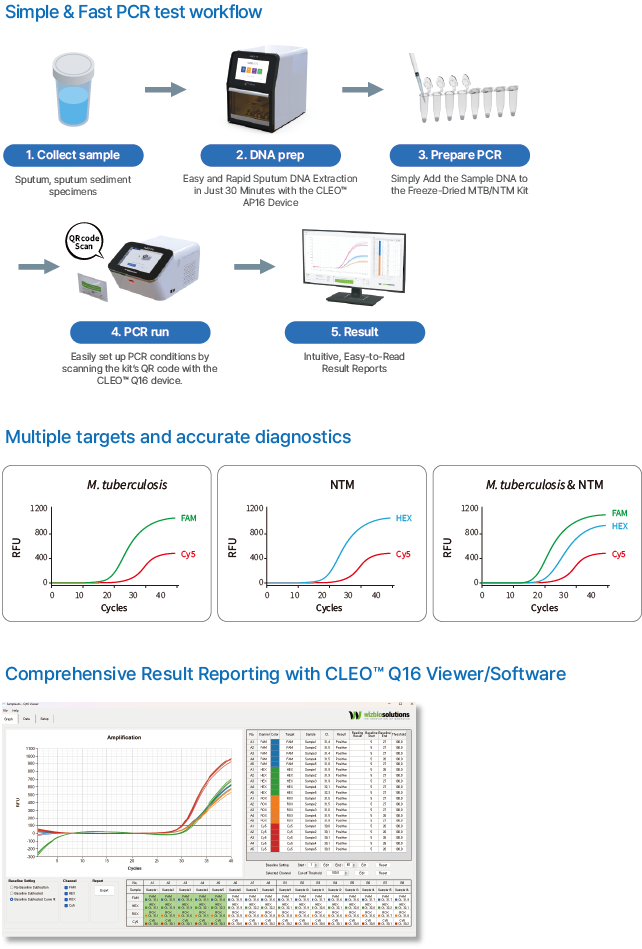
| Principle of Procedure |
Multiplex Real-time PCR |
| Target |
FAM for Mycobacterium tuberculosis (MTB), HEX for Non-tuberculosis mycobacteria (NTM), and Cy5 for the internal control |
| Tests for Package |
96 Tests/kit |
| Reagent Type |
Lyophilized (Freeze-dried) in a PCR tube |
| Limit of Detection (LoD) |
10 copies/rxn for Mycobacterium tuberculosis (MTB),
25 copies/rxn for non-tuberculosis mycobacteria (NTM) |
| Precision |
Respectively detect the reference specimen of high and low concentrations in different time ranges, 10 times, and the precision values of intra-assay and inter-assay Ct values were all <5%.. |
| Specificity |
No cross-reactivity of the assay was observed for Adenovirus, hCoV, Parainfluenza, RSV, SARS-CoV-2, Influenza virus, Legionella pneumophila, Mycoplasma pneumoniae, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, HPV, and HIV. |
| Sample Types |
Sputum, sputum sediment specimens. |
| Shipping/Storage |
Shipping and storage at room temperature for 12 months |